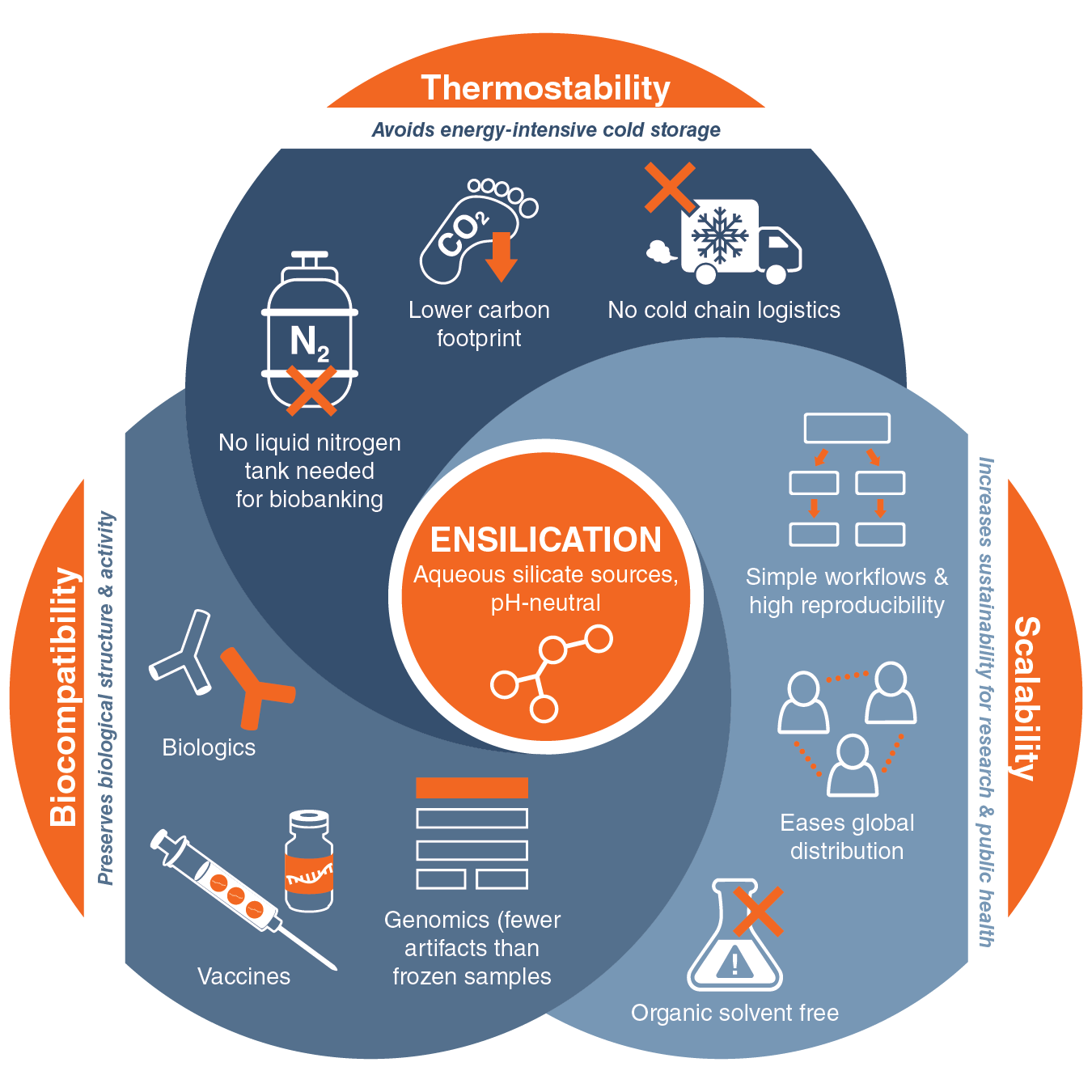
Ensilication can flip the script on nature by making biomolecule stocks thermostable, including complex biologics that are not amenable to lyophilization. Eliminating cold storage and transport requirements would boost sustainability for biomedical cold chains and biobanking. The potential waste and energy savings for clinical and research settings are tantalizing.
Cold-Storage is a process bottleneck in Research & Public Health
Currently, cold-chain temperatures are essential for vaccine banking, biobanking and many operations in research and clinical labs. It can be easy to forget that this system, established for decades, entails inherent risk and high infrastructure costs.
Cold storage generally prevents microbial growth; however, specific temperatures are required to preserve the integrity of biomolecules for specific uses. Vaccines present big cold storage challenges. Most standard lyophilized childhood vaccines require refrigeration at 2–8°C. Bulk antigen stocks held for extended periods for emergency vaccine production, and live-attenuated viral vaccines, such as varicella (chickenpox), must be stored frozen, at or below –15°C. RNA vaccines are more demanding. The lipid nanoparticles that encapsulate mRNA vaccines are easily damaged, cannot be lyophilized, and must be kept at ultra-low temperatures. A much wider range of biological sample types, including nucleic acids, cells, whole blood, and biopsied tissues, are stored in laboratories and large repositories known as biobanks. Specific cold temperature conditions are required to preserve sample integrity for each sample type and downstream analyses. Typically, a biobank is hosted by a hospital system or research organization to support clinical testing or a specific research focus. Large biobank repositories store hundreds of thousands to tens of millions of samples integrated with data. The world’s largest biobanks include UK Biobank, All of Us (U.S. NIH), BioBank Japan, Biobank Graz, Shanghai Zhangjiang Biobank, FinnGen, and the China Kadoorie Biobank. Given the expected extended frozen storage timeframes, many ultra-low-temperature freezers and liquid nitrogen tanks are involved. As you might suspect, cold storage is the main driver of energy consumption in biobanking. (1)
Demand for cold transport for biopharmaceuticals is increasing. The millions of biological samples circulating in research and healthcare join a growing number of pharmaceutical biologics in cold chain transport systems. The biologics category of drugs and therapies is expanding because they deliver improved patient outcomes. The catch-22 is that therapeutic recombinant proteins, antibodies, gene therapies and mRNA vaccines are less stable than small-molecule chemical drugs. Most regulatory-approved biologics are in liquid formats, which are manageable for clinics. Unfortunately, it’s very challenging, or even impossible, to lyophilize these complex therapeutics for ambient temperature storage — unlike many cytokines, enzymes, and other reagents commonly used in research, medical treatment or diagnostics. Storage at –20 °C or –80 °C can be necessary to prevent degradation that reduces medical effectiveness, and increases the risk of immunogenicity. Cold chains add complexity and risk loss.
In parallel to the growth of biologics, genomic analyses are also expanding. Genomic data provides valuable genetic insights and supports precision medicine. Next-generation sequencing (NGS) is fast, comprehensive, and highly sensitive. Sequencing methods are established research techniques that are increasingly automated at scale for discovery and diagnostics. As sequencing costs have come down, NGS is playing a key role in improving healthcare. NGS is already routinely used for rapid, accurate diagnosis or risk screening in oncology and clinical genetics. Oncologists use molecular profiling of tumors for diagnostic testing and planning treatment courses. Sequencing is also an important tool in pharmacogenomics and infectious disease.
Declining sequencing costs have raised the profile of cold storage infrastructure and transport for collected samples to NGS-capable labs. It’s an indirect economic barrier. For example, DNA is only stable for short periods at room temperature in a buffered solution. It will degrade, even when stored at −20 °C. Ultra-low temperatures of −80 °C are recommended for long-term storage. Liquid nitrogen is ideal for biobanking, enabling indefinite storage and supporting testing over months and years. Ultimately, the goal is to preserve the integrity of DNA samples from diseased patient populations over years to decades for follow-up and research purposes
Does anyone wonder if there is another way? Yes, cold storage is a growing sustainability concern. Cold storage systems exclude lower-resource point-of-care and research laboratory settings. It’s clear that an accessible method for ambient-temperature storage would expand access to research tools, precision medicine and life-saving biologics.
Ensilication is emerging with promising benefits
Ensilication is a solution-gelation-based chemistry that stabilizes biomolecules with a silica (SiO₂) coating. It’s reminiscent of how unicellular diatoms and glass sponges produce their protective glass-like cell walls.(2) It’s been around for some time. Excitement is growing because advances in ensilication chemistry offer many advantages.
Here is a high-level overview of how ensilication chemistry works. Precursor silica species carry negatively charged silanol groups (Si–OH). Early, commercialized, sol-gel chemistry relied on the toxic tetramethyl orthosilicate (TMOS) or Tetraethyl orthosilicate(TEOS) as the silicate precursor, heating, and harsh organic solvents to encapsulate molecules in silica. Working with hazardous precursors and organic solvents was not ideal. The chemistry has been refined to eliminate hazardous organic solvents, making it greener than the original approaches. Newer methods use aqueous silicate sources and bypass the hazardous chemistry of earlier silica sol-gel chemistry approaches. At room temperature, under controlled pH and ionic conditions, silica polymerizes on the surfaces of biomolecules via electrostatic attraction to their positively charged regions. Molecular structures are neither damaged at the nanoscale nor chemically modified. The process is highly reproducible. No freeze dryer or additional specialized lab equipment is required. These aspects make it suitable for processing individual lab stocks or for scaling up to high production of biologics.
Importantly, ensilication is reversible. Silica can be dissolved using fluoride-containing buffers or mild chemical treatments without harming the biomolecule, allowing the active molecule to be released intact.
Overall, ensilication can shield relatively simple molecules or complex biologics from desiccation, thermal stress and mechanical agitation. It is broadly compatible with biomolecules, including genetic material, antibodies, and vaccines. Ensilication is not a universal solution for room-temperature preservation of all biological sample types, yet its capabilities cannot be ignored. Protein Subunit and Conjugate Vaccines, vaccines with adjuvants and enzymes that can’t be lyophilized, have been successfully encapsulated. DNA and RNA can be encapsulated in silica matrix mechanisms (3,4). Higher sample integrity, improved thermostability, and user-friendly protocols, compared with lyophilization, would certainly benefit next-generation sequencing. Ensilication is an all-around exciting proposition for the life sciences.
Ensilication techniques are being optimized for real-world use cases. Based on publicly available information, it's likely that specific stock types, workflow durations, and retrieval conditions are being targeted. For example, EnsiliTech® is a spin-out from the University of Bath that uses a patented formulation to enable the transport of biomolecules at temperatures up to 50°C. The team is targeting therapeutic and diagnostic biomolecules. A series of investigations by researchers at the University of Bath, the University of Newcastle, the University of Duhok, and Ensilitech demonstrated the biocompatibility and feasibility of ensilication across a range of samples. (5,6,7) Ensilitech has produced sufficient evidence to secure government funding to focus on thermostable vaccines and biologics. Word to the wise. According to their website, they are also seeking partners to further develop these technologies and establish performance benchmarks for a range of biomolecules. In another example, researcher Mónica López Fanarraga, of the Valdecilla Health Research Institute (IDIVAL) and the University of Cantabria, is collaborating with the Valdecilla Biobank to develop and validate patented DNA ensilication technology. (8)
Decisions to move away from frozen stocks will depend on reliable scientific data, practicality in lab work, and a positive return on the upfront investment. Thermostable vaccines alone would constitute a significant advance for pharmaceutical companies seeking to support sustainability in healthcare. Approximately 50% of vaccine stocks are wasted each year due to temperature-control logistical challenges and a lack of reliable electricity supply in healthcare facilities.(9) Affordable DNA ensilication kits could extend modern genomic testing to remote locations, developing nations, and point-of-care settings. Greener chemistry, lower costs, and better preservation are an invitation to scientific collaboration!